Industry Leaders Partner to Create a NEW Robotic Navigation Development Kit — Fast-Tracking New Applications to Market
Developing a medical robot with the dual ability to track a patient and robot dynamically with an optical tracker involves highly technical robotic and navigational tasks.
The KUKA Development Kit provides a solution for start-up and scale-up OEMs looking to save months in the development phase.
Integrate the KUKA Development Kit with your OEM medical device, and you’ll save time and costs otherwise spent on:
- Writing code for the robot and camera.
- Developing a system architecture and testing the flow of information.
- Developing a surgical cart including a robot base and stand.
- Securing regulatory body clearance and certifications.

Download the KUKA Case Study to learn more about our NEW Development Kit
By submitting this form, I acknowledge I’ve reviewed and accepted NDI’s privacy policy.
Industry Leaders KUKA, NDI, and MPE Partner on New Robotic Navigation Development Kit, Fast-Tracking Applications to Market
Start-up medical device OEMs need to develop quality medical technologies
at low risk and cost, and they need to do it fast.
Kick Start OEM Product Development with Low Risk
When NDI and KUKA first connected and started sharing some of the challenges their customers faced in getting their products to market, they recognized that there was a way to help. From here the collaboration to create a development kit was born.
Developing a medical robot with the dual ability to track a patient and robot dynamically with an optical tracker (“camera”) involves highly technical robotic and navigational tasks. Executing these tasks requires a significant investment of time and money from start-up and scale-up medical device OEMs (“customers”).
Historically, OEMs would be responsible for determining how to track the robot with the camera as well as the patient. This involves:
- Designing optical marker tools and determining how to mount them to the robot.
- Writing code for the robot and camera, with two separate coordinate systems and conventions.
- Developing a system architecture and testing the flow of information.
- Developing a surgical cart including a robot base and stand for the camera and displays.
- Securing regulatory body clearance and certifications.
These tasks come at a cost, including an OEM’s engineering overhead, coding cost, and time. By removing these arduous tasks, the development kit can save start-up customers several months in the development phase.
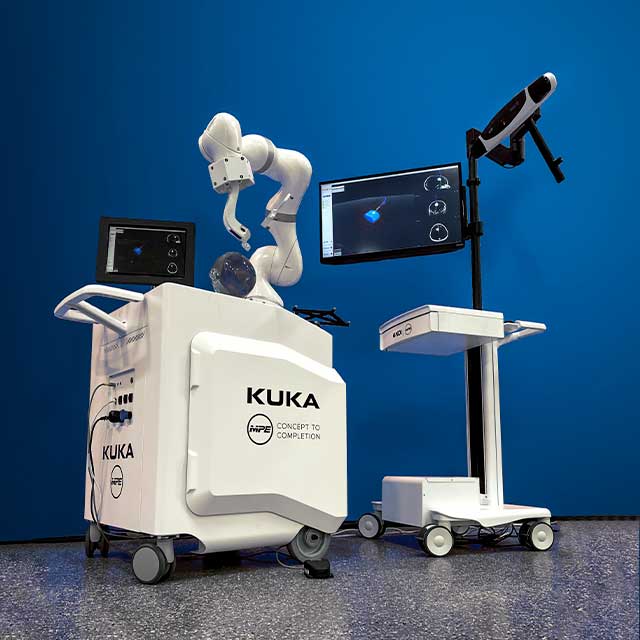
Navigate with Confidence
The KUKA robot development kit, born out of a partnership among global industry leaders KUKA, NDI, and MPE, provides OEMs with a low-risk, convenient code-based tracking solution to overcome these hurdles, accelerating an OEM’s time to market.
KUKA, a world leading robotics manufacturer, has partnered with NDI to produce a combined robot/optical tracking navigation development kit. The kit bundles together the KUKA LBR Med 14 R820 (“LBR Med”) collaborative robot (“cobot”), NDI Polaris Vega® XT optical tracker, and sample code, and is professionally packaged with a sleek modular medical cart designed by MPE that optimizes medical workflows.
The KUKA development kit is ideal for the early stages of OEM product design and prototyping. The kit enables OEM start-ups and scale-ups to fast-track early usability studies, technical design requirements, and bench-top testing phases. Customers can have confidence that they will accomplish proof of concept projects quickly to prepare for the next milestone, without regulatory hiccups. NDI, KUKA, and MPE have the regulatory certifications required to meet medical device, software, collaborative robot, and design control standards (see Appendix A).
Customers need to focus their efforts on clinical workflows and demonstrating the clinical value of their approach to potential stakeholders, versus consuming valuable resources integrating hardware components. Thankfully, with access to KUKA, NDI, and MPE’s integration and applications teams, customers can quickly integrate the KUKA development kit with their medical device to develop professional prototype product offerings at low risk.
Here’s How the KUKA Development Kit Delivers Innovative Functionality
Customers can easily leverage the KUKA development kit for their OEM-specific applications. Here’s the functionality customers can expect from the kit:
- Register and synchronize the KUKA LBR Med robot to the NDI Polaris Vega XT coordinate system.
- Track the KUKA LBR Med robot in real-time, using a multi-face end effector with retroreflective markers.
- Develop trackable customer accessory tools that can be optimized for customer-specific robotic surgery procedures.
- Adjust the robot path in real-time based on tool and patient position (“dynamic tracking”).
- Identify major software elements including communication protocols, user interface, and logic for applying NDI position data to move the robot.
KUKA, NDI, and MPE understand the importance of early engagement with start-ups and scale-ups. With access to KUKA, NDI, and MPE’s integration and application teams, customers can confidently kickstart the development of their unique medical device, integrating the low-risk, convenient KUKA development kit robot into their system.
Over 210 Years of Combined Experience
Industry leaders KUKA, NDI, and MPE share a customer-first philosophy and commitment to delivering innovative and quality solutions. This is evidenced by their strong research and development (R&D), integration, and product support teams, who are ready and willing to assist their medical technology partners throughout their product lifecycles. As authorities in their respective fields, KUKA, NDI, and MPE each bring their unique expertise and knowledge to this powerful collaboration.
Navigate with Confidence
The KUKA robot development kit, born out of a partnership among global industry leaders KUKA, NDI, and MPE, provides OEMs with a low-risk, convenient code-based tracking solution to overcome these hurdles, accelerating an OEM’s time to market.
KUKA, a world leading robotics manufacturer, has partnered with NDI to produce a combined robot/optical tracking navigation development kit. The kit bundles together the KUKA LBR Med 14 R820 (“LBR Med”) collaborative robot (“cobot”), NDI Polaris Vega® XT optical tracker, and sample code, and is professionally packaged with a sleek modular medical cart designed by MPE that optimizes medical workflows.
The KUKA development kit is ideal for the early stages of OEM product design and prototyping. The kit enables OEM start-ups and scale-ups to fast-track early usability studies, technical design requirements, and bench-top testing phases. Customers can have confidence that they will accomplish proof of concept projects quickly to prepare for the next milestone, without regulatory hiccups. NDI, KUKA, and MPE have the regulatory certifications required to meet medical device, software, collaborative robot, and design control standards (see Appendix A).
Customers need to focus their efforts on clinical workflows and demonstrating the clinical value of their approach to potential stakeholders, versus consuming valuable resources integrating hardware components. Thankfully, with access to KUKA, NDI, and MPE’s integration and applications teams, customers can quickly integrate the KUKA development kit with their medical device to develop professional prototype product offerings at low risk.
KUKA
KUKA is a world-leading supplier of intelligent automation solutions with 124 years of experience in multiple global sectors. KUKA uses the LBR Med robot in its partnership with NDI and MPE. The LBR Med is a safe, flexible seven-axis lightweight robot, that’s been specially developed as a component for the medical market. The 7th axis enables the robot to manipulate the shape of the arm around the surgical space, facilitating workflow and minimizing line of sight issues.
Equipped with redundant integrated torque sensors, the LBR Med has strong haptic capabilities, enabling it to perceive external forces and have safe collision detection. The LBR Med robot deals with highly sensitive tasks with varying parameters and can be seamlessly integrated into a medical device and adapt flexibly.

NDI
NDI, an industry leader with over 40 years of expertise and innovation in optical and electromagnetic navigation technology, integrates its Polaris Vega® XT optical navigation system with the KUKA’s LBR Med robot.
The Polaris Vega XT combines unrivaled measurement accuracy, high-speed tracking (400Hz), and low latency (< 3ms), facilitating the seamless integration of 3D tracking within OEM robotic surgical systems. Here’s how the Polaris Vega XT works:
A reference tool with reflective markers is placed near or affixed to the patient, establishing a reference location against which surgical instruments are tracked. These same markers are attached to the surgical instruments. Infrared light (IR) from the optical tracker floods the measurement volume and illuminates the reflective markers. Light from the markers reflects to IR sensors on the optical tracker, determining the coordinates of the markers, and the position and orientation of the surgical instrument.
The integration of NDI’s Polaris Vega XT with KUKA’s LBR Med robot gives rise to a KUKA development kit that can receive patient and instrument position and orientation data. With these data, the development kit can track a patient and robot dynamically with precision, accuracy, and speed. Customers can benefit from these features once the development kit is integrated into their medical device.
The Polaris Vega XT and KUKA LBR Med robot are integrated into MPE’s medical cart, resulting in a product that is market-ready for OEM integration.
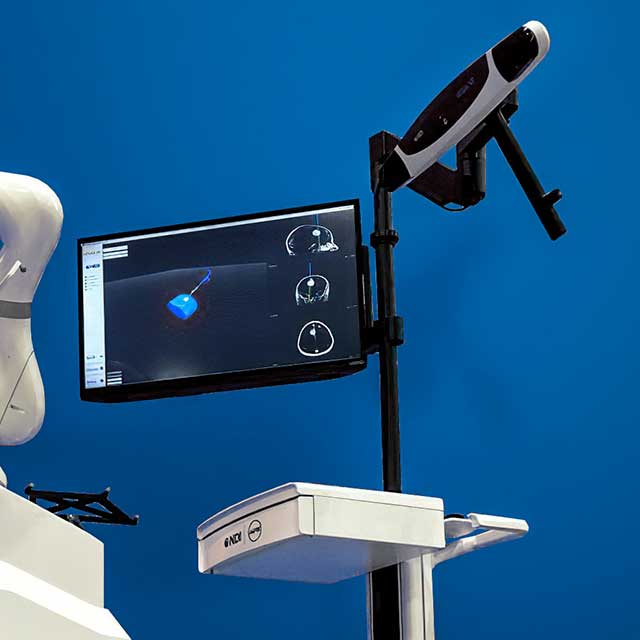
MPE
MPE is the largest custom medical device cart designer and manufacturer in the world with over 44 years of expertise. Their best-in-class design and manufacturing experience means the KUKA robot cart and camera stand are professionally finished and optimized for human ergonomics and can be integrated to fit a customer’s desired medical workflow. MPE can customize the KUKA robot’s cart and/or camera stand to maximize clinical utility for a customer’s unique medical device.
MPE’s product development process aligns with best manufacturing practices, materials, and regulatory compliance to reduce costs and risks for start-up and scale-up OEMs. As an OEM presenting your new offering to potential stakeholders, your medical device needs to be a scalable market-ready product that can roll right into the operating room. In collaboration with KUKA and NDI, MPE streamlines this process, giving the OEM the best opportunity for market launch success.
KUKA, NDI, and MPE’s development kit works seamlessly right out of the box. The last step is integration and customization for your unique OEM medical device.

Download the Case Study: Industry Leaders Launch Robotic Navigation Kit to Fast-Track Market Applications
Discover how the KUKA Development Kit can revolutionize the development of your medical robot. Save months in the development phase and reduce costs by integrating this kit with your OEM device. Eliminate the need to write code, develop system architecture, and create a surgical cart from scratch. Plus, it benefits from pre-secured regulatory clearances. Read our case study to see how you can fast-track your innovations and bring medical solutions to market faster.
