Polaris Vega® ST
Accurate and reliable optical navigation for surgical tool tracking and navigated applications.
Versatile Optical Tracker for Diverse OEM Surgical Applications
The Polaris Vega ST is a flexible optical tracker that can provide trusted measurements for OEM-supported surgical applications. Includes Radiation Hardening option for safe integration into radiation therapy suite workflows.


Enhanced Radiation Protection
The Polaris Vega ST offers an exclusive Radiation Hardening option, protecting the optical tracker from gamma and neutron radiation. This added protection allows safe integration into OEM surgical navigation systems and radiation therapy suite workflows.
Robust Design, Exceptional Accuracy,
and Streamlined Integration.

Robust Hardware Design
Optional radiation hardening ensures reliable performance in harsh environments like radiation therapy suites.
Exceptional Measurement Accuracy
A volumetric accuracy to 0.12 mm RMS with low noise enables accurate and reliable tool tracking.
Streamlined Tool Design
Built-in API and available software enables tool development and accelerated integration into OEM software applications.
Polaris Vega ST Technical Specifications:
| PERFORMANCE | |
|---|---|
| Frame Rates | 20, 30, 60 Hz |
| Average Latency | 17 ms (typical) at 60 Hz |
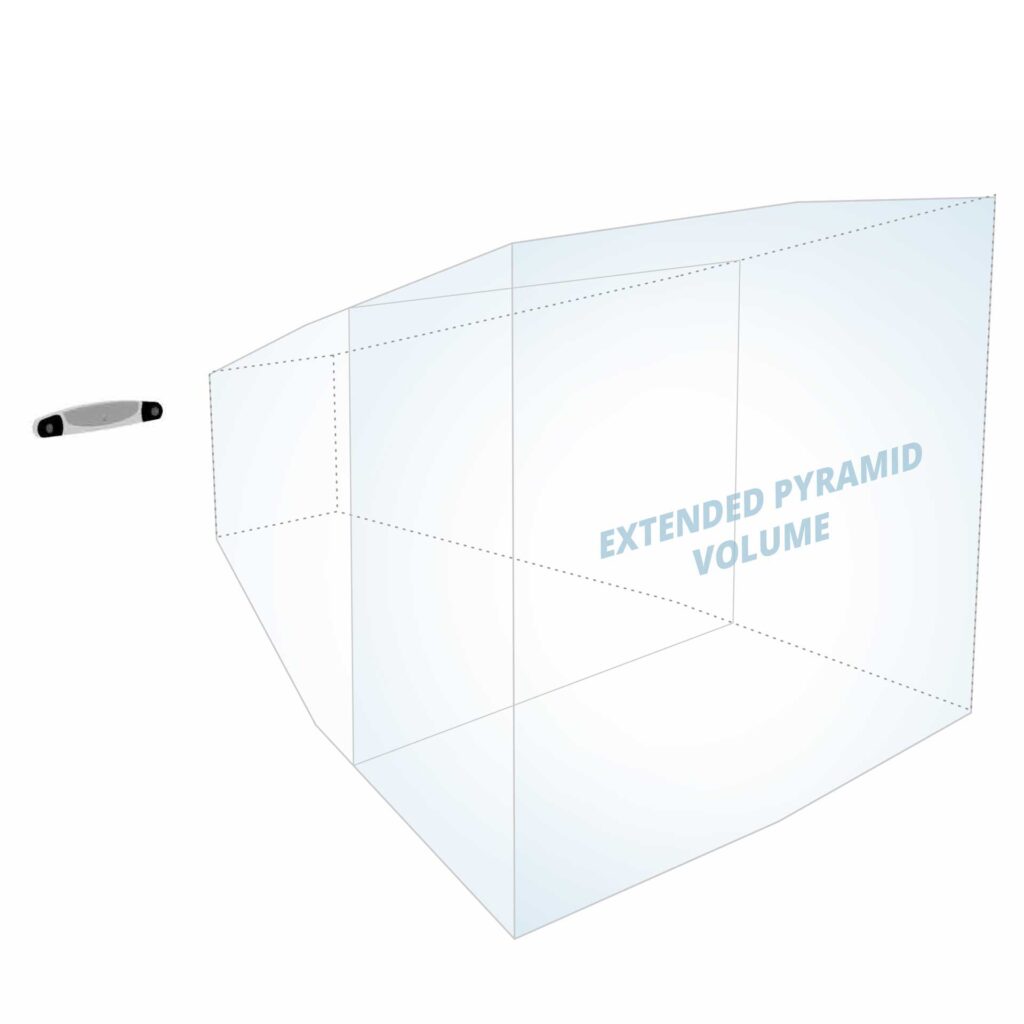
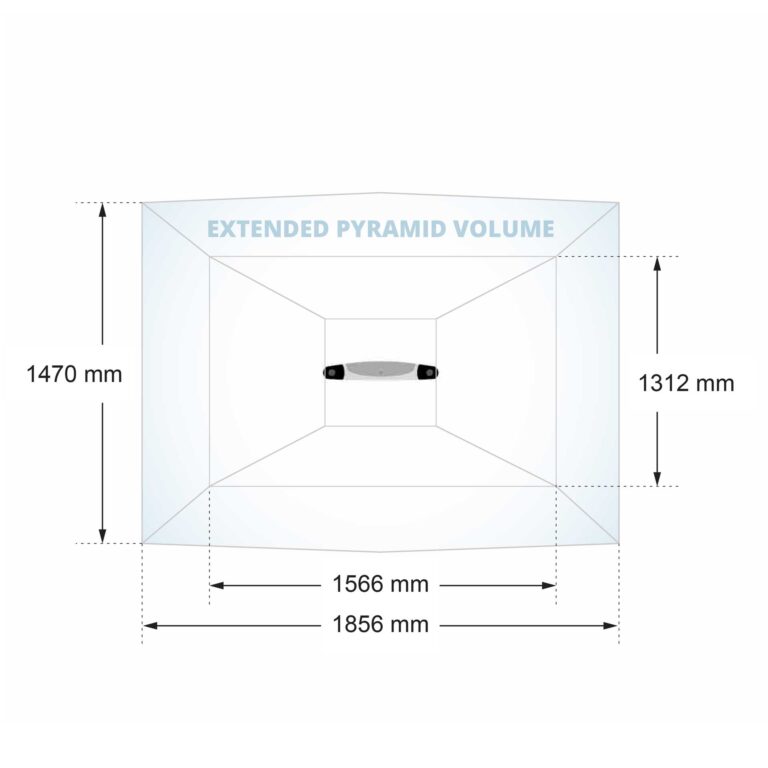
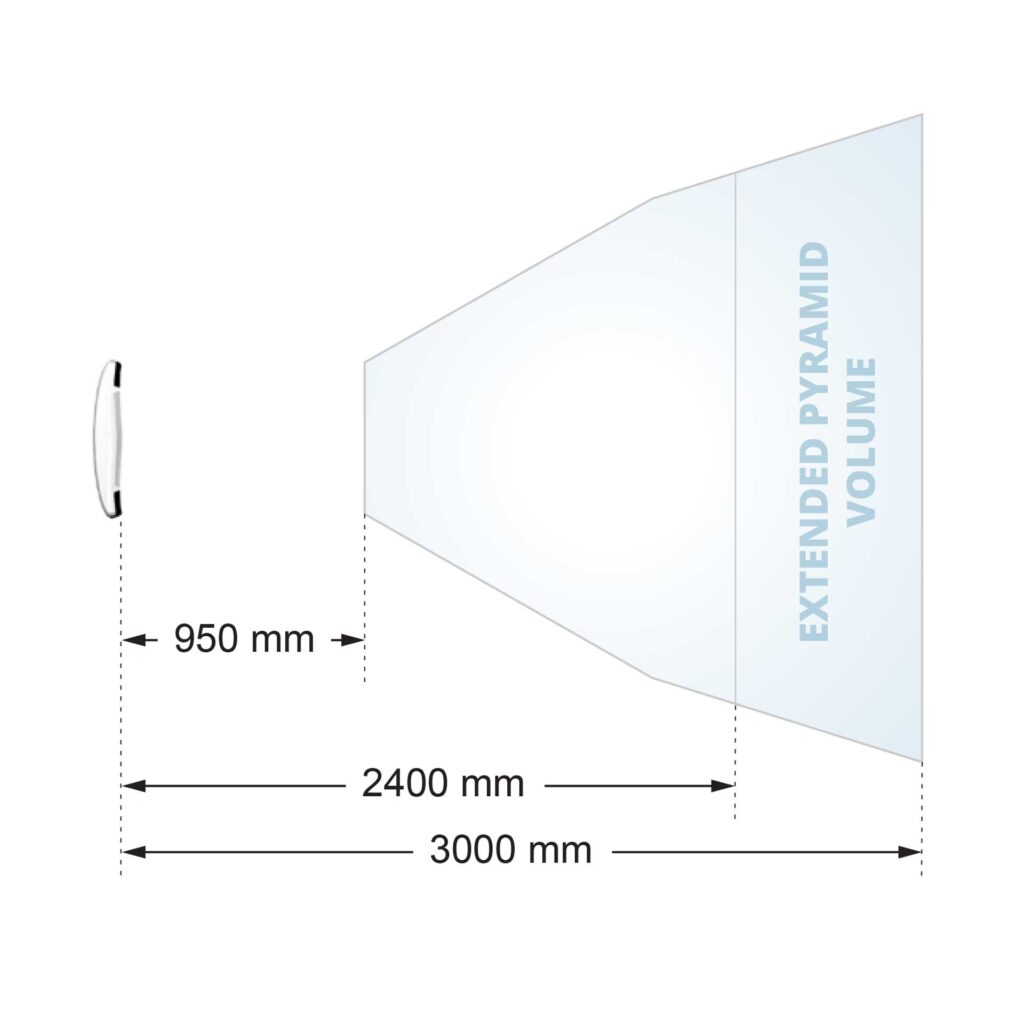
| Measurement Volume | Pyramid Extended Pyramid (optional) |
| Volumetric Accuracy1,2 RMS | Pyramid: 0.12 mm Ext. Pyramid: 0.15 mm |
| 95% Confidence Interval1,2 RMS | Pyramid Volume: 0.20 mm Extended Pyramid: 0.30 mm |
| TOOL TRACKING | |
| Tool Types | Passive, Active Wireless |
| Maximum Number of Tools | Load up to 25 tools (maximum of 6 active wireless) |
| Maximum Number of Markers per Tool | 6 single-face/20 multi-face |
| DATA COMMUNICATION & CONNECTIVITY | |
| Data Communication | Gigabit Ethernet |
| Communication Protocol | TCP |
| Data/Power Interface | Ethernet, RJ45 |
| HARDWARE | |
| Dimensions (L x W x H) | 591 x 103 x 106 mm |
| Radiation Hardening | Optional |
| Weight | 1.7 kg 2.15 kg (with Radiation Hardening) |
| Mounting | Four M4 x 0.7 mm pitch x 10 mm deep threaded holes, rear mount |
1 Based on a single marker stepped through more than 900 positions throughout the measurement volume using the mean of 30 samples at each position at 20°C.
2 Accuracy stated based on overall volume.

Download the
Polaris Vega ST Datasheet
Precision and dependable optical guidance for surgical instrument tracking and navigational uses.
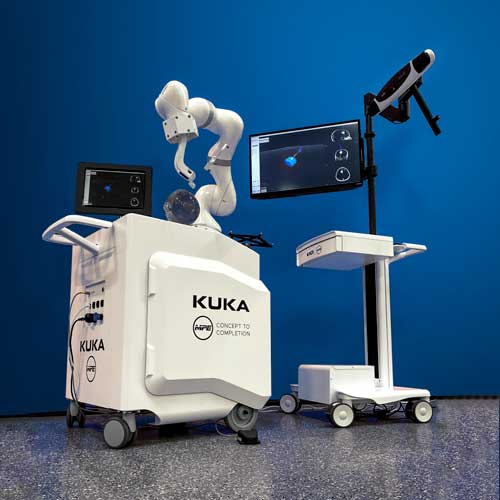
Customization and Integration Options
Tailor NDI’s optical navigation solutions to your unique tracking application with diverse customization and integration options.

Download the
Optical Education Guide
Download this 14-page guide to learn more about optical navigation technology and the advantages it can provide for OEM solution providers.
Our Versatile Optical Tracker for Diverse OEM Surgical Applications
The Polaris Vega® ST is a versatile optical tracker that can provide trusted measurements in an array of OEM-supported surgical applications, delivering accurate and reliable 3D tracking data. Tracking is achieved via navigation markers attached to OEM surgical instruments such as probes, shavers, awls, drills, microdebriders, and more.
Exclusive to the Polaris Vega ST is an available Radiation Hardening option. The Radiation Hardening option protects the optical tracker from gamma and neutron radiation through a shielded processor and modified memory management. This extra protection allows the Polaris Vega ST to be safely integrated into OEM surgical navigation systems and workflows used in radiation therapy suites.
More customization options are available to enhance system utility and flexibility. The Positioning Laser assists with positioning the optical tracker and/or surgical equipment within the measurement volume. The Extended Pyramid Volume nearly doubles the measurement volume, which benefits procedures requiring a clear view of several surgical instruments simultaneously.
Exceptional Measurement Accuracy
The Polaris Vega ST delivers high volumetric accuracy to 0.12 mm RMS with low noise. Track passive spheres attached to tools with confidence; hardware characterization and factory calibration optimize accuracy for measurements that are highly repeatable and reliable.
| Volumetric Accuracy1,2 RMS | Pyramid Volume: 0.12 mm Extended Pyramid: 0.15 mm |
|---|---|
| 95% Confidence Interval1,2 | Pyramid Volume: 0.20 mm Extended Pyramid: 0.30 mm |
Robust Hardware Design
The Polaris Vega ST can include optional radiation hardening to maintain accurate and reliable performance in harsh environments, such as those found in radiation therapy suites. Consistent operation in variable room conditions adds to product robustness.
| Measurement Rates | 20, 30, 60 Hz |
|---|---|
| Average Latency | 17 ms (typical) at 60Hz |
| Measurement Volumes | Pyramid, Extended Pyramid (optional) |
Streamlined Tool Design
The Polaris Vega ST has a built-in API and available software to facilitate tool development and accelerate integration into OEM and end-user software applications. The NDI ToolBox software includes utilities for ongoing system diagnostics and maintenance.
| Tool Types | Passive, Active Wireless |
|---|---|
| Maximum Number of Tools | Load up to 25 tools (maximum of 6 active wireless) |
| Maximum Number of Markers per Tool | 6 single-face/20 multi-face |
Ethernet Connectivity
The Polaris Vega ST comes with Gigabit Ethernet capable of streaming high-speed tracking data back to the OEM host application. The Ethernet connection also provides power to the Vega and allows for greater flexibility and interoperability of equipment within the operative space.
| Data Communication | Gigabit Ethernet |
|---|---|
| Maximum Number of Tools | Network Synchronization |
| Data/Power Interface | Ethernet, RJ45 |
Legal Disclaimer
NDI tracking and measurement products are general metrology components that can be integrated into customer products, research experiments, and/or as components of medical devices that require precision measurement and tracking. While NDI components and technology can be integrated into original equipment manufacturer (OEM) medical devices, they are not specifically intended for a given application and, as such, have not been developed or manufactured in accordance with medical device standards. It remains the responsibility of the OEM customer or end-user to determine and test the suitability of NDI components and technology for their intended use, including performing any required ethics approval, verification, and validation required to demonstrate suitability and compliance. System-level testing, certification, and validation are the responsibility of the original equipment manufacturer or the applicable end-user and should be completed prior to the use of NDI products or technologies in any application.