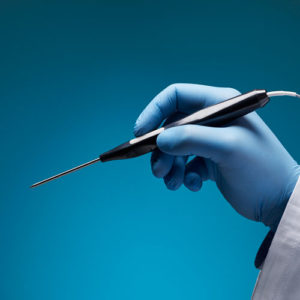
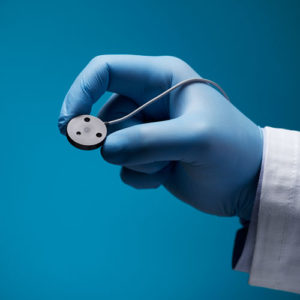
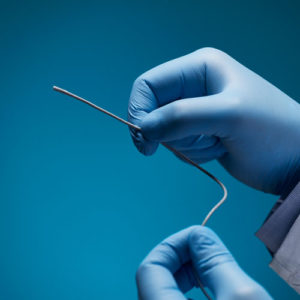
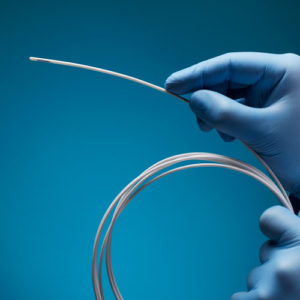
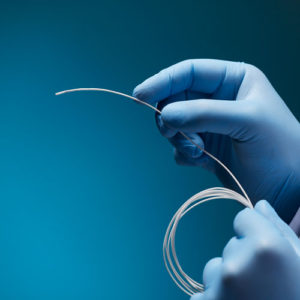
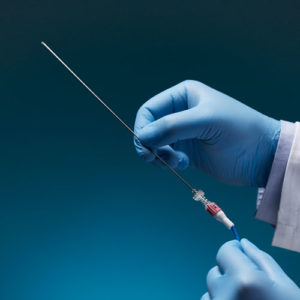
Aurora Ready-to-Use Tools
For quick and easy tracking during the exploratory stages of OEM development, we offer six ready-to-use tools, each embedded with a 5DOF or 6DOF sensor.
No Tool Assembly, Configuration or Characterization Required
Aurora® ready-to-use tools include everything required to begin tracking right out of the box. Designed with speed and ease of integration and use in mind, these tools are an excellent introduction to the Aurora electromagnetic tracking solution during research applications and the exploratory phase of OEM research and development. No assembly, configuration, or calibration are required. (Note: Aurora ready-to-use tools are not intended for medical use.)
Features:
- Comes wired to a connector and programmed for immediate use.
- Start tracking without having to configure or calibrate the tool.
- Available in a variety of formats for diverse tracking applications.
Download our 12-page Education Guide to learn how you can integrate Electromagnetic Tracking Technology into your OEM medical devices to:
- Navigate instruments safely and reliably through complex anatomy.
- Target small treatment areas with
sub-millimeter accuracy and precision. - Visualize real-time position as well as orientation of an instrument.
- Track instruments even when they
are out of sight. - Embed micro sensors into flexible and
rigid instruments.

Legal Disclaimer
NDI tracking and measurement products are general metrology components that can be integrated into customer products, research experiments, and/or as components of medical devices that require precision measurement and tracking. While NDI components and technology can be integrated into original equipment manufacturer (OEM) medical devices, they are not specifically intended for a given application and, as such, have not been developed or manufactured in accordance with medical device standards. It remains the responsibility of the OEM customer or end-user to determine and test the suitability of NDI components and technology for their intended use, including performing any required ethics approval, verification, and validation required to demonstrate suitability and compliance. System-level testing, certification, and validation are the responsibility of the original equipment manufacturer or the applicable end-user and should be completed prior to the use of NDI products or technologies in any application.