Aurora®
Our premier electromagnetic tracking solution, which combines unrivalled measurement accuracy and expert customization for the most intricate OEM interventional applications.
Minimally Invasive Procedures with Electromagnetic Tracking Technology
The Aurora® electromagnetic tracking solution provides unobstructed real-time tracking of micro sensors that can be embedded into rigid and flexible OEM medical instruments such as ultrasound probes, endoscopes, catheters, guidewires – even at the tip of a needle. With the Aurora® electromagnetic tracking solution, no line-of-sight is needed for in-vivo tracking of instruments through twisting anatomical tracts.
When integrated as a component into the workflow of OEM image-guided surgery or interventional systems, the Aurora acts as the link between patient image sets and 3D space, enabling the instruments’ positions and orientations to be instantly localized and visualized within the operative field. It does so with the exceptional speed, accuracy, and precision required by today’s most demanding minimally invasive approaches.

Aurora Core Components
The Aurora electromagnetic tracking solution consists of the following four core components that work together to deliver real-time OEM instrument tracking capabilities. (Read about How Electromagnetic Tracking Works for more details.)

System Control Unit (SCU)
Controls the field generator, collects information from the SIUs, calculates the position and orientation of each sensor, and interfaces with the host computer. Both PCB and enclosed formats are available, which can be seamlessly integrated with standalone applications and OEM carts.
Dimensions (enclosed format): 84 x 172 x 230 mm
Weight (enclosed format): 2.0 kg

Sensor Interface Unit (SIU)
Amplifies and digitizes the signals from the sensors. Up to two SIUs can be connected to a single SCU. Available with 2, 4, 6, or 8 ports; each port can host one 6DOF sensor/tool or two 5DOF sensors/tools.
Dimensions (enclosed format): 53 x 172 x 114 mm
Weight (enclosed format): 660 g

Field Generator (FG)
Emits a low-intensity, varying electromagnetic field and establishes the position of the measurement volume. There are five Aurora FGs, each with distinct measurement volume, form factor, and mounting options. The FGs feature plug-and-play functionality with the System Control Unit.
Dimensions (for Planar 20-20 FG): 200 x 200 x 71 mm
Weight (for Planar 20-20 FG): 2.6 kg
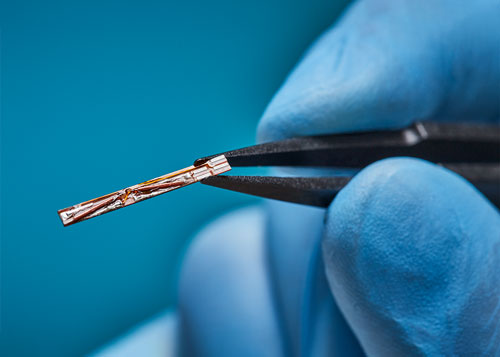
Sensors
Act as localization points within the measurement volume, with position and orientation data relayed to the host interface for visualization. Sensors can be embedded into OEM medical instruments such as ultrasound probes, scopes, catheters, guidewires – even at the tip of a needle.
Number of Standard Sensors: 4 6DOF and 5 5DOF**
Maximum Number of Tracked Sensors: 16 6DOF or 32 5DOF
Aurora Technical Specifications
AuroraCube Volume | AuroraDome Volume | |||
|---|---|---|---|---|
| RMS | 95% CI | RMS | 95% CI | |
| Accuracy – 5DOF Sensor*†‡ | ||||
| Position | 0.70 mm | 1.40 mm | 1.10 mm | 2.00 mm |
| Orientation | 0.20° | 0.35° | 0.20° | 0.40° |
| Frequency | 800 Hz | 800 Hz | ||
| Measurement Rate | 40 Hz | 40 Hz | ||
| Accuracy – 6DOF Sensor*†‡ | ||||
| Position | 0.48 mm | 0.88 mm | 0.70 mm | 1.40 mm |
| Orientation | 0.30° | 0.48° | 0.30° | 0.55° |
| Frequency | 800 Hz | 800 Hz | ||
| Measurement Rate | 40 Hz | 40 Hz | ||

3D Guidance®
Our quality electromagnetic tracking solution, which features ready-to-integrate solution components and sensors for rapid OEM product development and time to market.
Download our 12-page Education Guide to learn how you can integrate Electromagnetic Tracking Technology into your OEM medical devices to:
- Navigate instruments safely and reliably through complex anatomy.
- Target small treatment areas with
sub-millimeter accuracy and precision. - Visualize real-time position as well as orientation of an instrument.
- Track instruments even when they
are out of sight. - Embed micro sensors into flexible and
rigid instruments.

Legal Disclaimer
NDI tracking and measurement products are general metrology components that can be integrated into customer products, research experiments, and/or as components of medical devices that require precision measurement and tracking. While NDI components and technology can be integrated into original equipment manufacturer (OEM) medical devices, they are not specifically intended for a given application and, as such, have not been developed or manufactured in accordance with medical device standards. It remains the responsibility of the OEM customer or end-user to determine and test the suitability of NDI components and technology for their intended use, including performing any required ethics approval, verification, and validation required to demonstrate suitability and compliance. System-level testing, certification, and validation are the responsibility of the original equipment manufacturer or the applicable end-user and should be completed prior to the use of NDI products or technologies in any application.
* Volumetric accuracy. All data collected with the Aurora V3 System in an environment free of electromagnetic disturbances.
** More Available
† Typical results. The accuracy of specific sensors will vary.
‡ Accuracy depends on tool design and the presence of metal. Note: Results based on more than 1000 random positions and orientations distributed throughout the characterized volume.